Macrocyclic Metal Complexes - Phthalocyanines
Phthalocyanines
Phthalocyanine is a planar 18 -electron heterocyclic aromatic
system derived from porphin. The more systematic name is [therefore]
tetraazatetrabenzoporphyrine. The annullation of further benzene units leads to
the naphthalocyanines 1,2-NcH
2
and 2,3-NcH
2
, respectively
(figure 1).
-electron heterocyclic aromatic
system derived from porphin. The more systematic name is [therefore]
tetraazatetrabenzoporphyrine. The annullation of further benzene units leads to
the naphthalocyanines 1,2-NcH
2
and 2,3-NcH
2
, respectively
(figure 1).
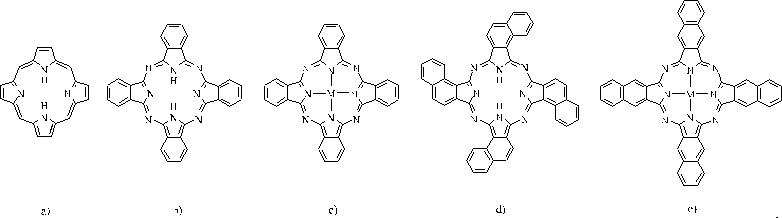
Abb.1
a.)Porphin, b.)PcH
2
, c.)PcM, d.)1,2-NcH
2
(C
4h
-Isomer), e.) 2,3-NcM
A large variety of complexes of the planar macrocycle Pc
2-
and its
derivatives have been described in the literature [1]
. Main group metals (e.g.Si, Ge) as well as numerous transition metals like
Fe, Co or Ni serve ascentral units.
Research Areas
Due to their macrocyclic nature including extended
 -systems,
phthalocyanines and naphthalocyanines are capable of forming organic
conductors. Charge transport is enabled by specific orientation, which can be
enhanced by doping, e.g. with iodine. One distinguishes between planar, stacked
[2]
and axially bridged
[3]
polymer systems (figure 2).
-systems,
phthalocyanines and naphthalocyanines are capable of forming organic
conductors. Charge transport is enabled by specific orientation, which can be
enhanced by doping, e.g. with iodine. One distinguishes between planar, stacked
[2]
and axially bridged
[3]
polymer systems (figure 2).
Some years ago, we developed a method for the transformation of
phthalocyaninato- and naphthalocyaninato-transition metal compounds into
coordination polymers as shown in figure2. The bridging is effected by neutral
ligands with sigma-donor ability such as pyrazine (pyz), tetrazine (tz),
diisocyanobenzene (dib) etc. In this macrocycle system, central transition
metal atom and the bridging ligand can be systematically varied to influence
the physical properties such as conductivity, nonlinear optical properties and
others.
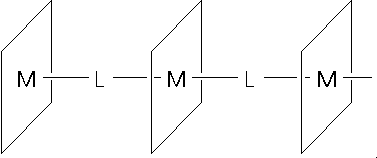
 = Pc, 1,2-Nc, 2,3-Nc, TBP, TPP
= Pc, 1,2-Nc, 2,3-Nc, TBP, TPP
M = transition metal (+II, +III), e.g. Fe, Ru, Os, Co, Rh, Mn, Cr
L = pyz, tz, bpy, dib, me
2
dib, me
4
dib, CN
-
SCN
-
Abb.2
Axiallly bridged Macrocyclics
These bridged macrocyclic metal complexes (shish-kebab polymers) can be
obtained in high yield and purity. Due to the choice of adequate bridging
ligands, e.g. tetrazine, shish-kebab polymers of the structural type depicted
in figure 2 display intrinsic semiconducting properties, while on the other
hand chemical and electrochemical doping are still possible without destroying
the polymer structure. Bridged systems with central metal atoms in the
oxidation state +3 (e.g. Fe
3+
, Co
3+
, Rh
3+
) are
obtained with CN
-
SCN
-
or N
3-
. The preparatively easily accessible
µ-cyano-polymer [PcCo(CN)]
n
, which displays good semiconducting
properties with
 RT
= 2·10
-2
S/cm, without
external doping should be mentioned here[3]
.
RT
= 2·10
-2
S/cm, without
external doping should be mentioned here[3]
.
A more recent issue amongst polymeric metal macrocycles are the ladder
polymers. This concept is realized by the synthesis of monomeric macrocycles
with D
2h
-symmetry capable of Diels-Alder reactions, such as the
hemiporphyrazines, and their reactions with dienes and dienophiles (Scheme
1).[4]
.
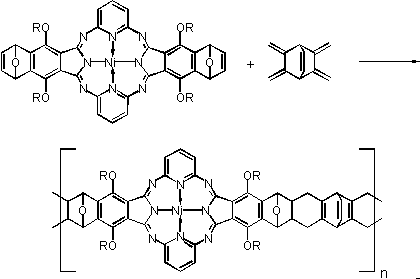
Scheme 1:
Synthesis of a ladder polymer on Hemiporphyrazine Basis
According to Scheme 1, syntheses of ladder polymers on phthalocyanine basis are
also being investigated. To this end, investigations are under progress to
synthesize phthalocyanines with D
2h
-symmetry capable undergoing Diels-Alder
reaction are synthesized and reacted with dienes and dienophiles to form ladder
polymers. The double bonds required for conjugation are introduced into the
obtained ladder polymers chemically.
Due to the fact that the D
2h
phthalocyanines depicted in Scheme 1 are
unsymmetrically substituted phthalocyanines, our research group is intensivly
working on the separation of such substituted phthalocyanines: peripherally
tetrasubstituted phthalocyanines are always obtained as a mixture of the four
possible constitutional isomers (D
2h
, C
2v
, C
4h
, C
s
Their definite analyticaland preparative separation and characterization by nuclear
magnetic resonance was achieved for the first time by us recently
[5]
.
Further phthalocyaninato-metal compounds such as PcTiO and peripherally
substituted soluble derivatives are at present investigated for their
photoconducting properties.
Some tetra- and octasubstituted phtahlocyanines show liquid crystalline
behaviour depending on their substituents, which are being studied for their
suitability as Langmuir-Blodgett films andas optical limiting compounds.
Phthalocyaninatometal compounds and comparable macrocyclic metal complexes are
prepared for molecular pattern recognition and their sensory properties
investigated. Phthalocyaninato-silicon complexes bridged by acetylene
[PcSiC
2
n
are synthesized for use in the construction of nanostructures.
Water-soluble phthalocyaninato Zinc complexes of different structures and with
different substituent patterns are synthesized and tested systematically for
their application potentials in photodynamic cancer therapy.
Certain tetra- and octasubstituted phthalocyaninatometal complexes embedded in
suitable polymer films display positive effects in the gas separation of
nitrogen and oxygen. The main aspect of research here is the dependence of this
property on the central metal atom and the peripheral substituents.
Recently, some transition metal phthalocyanines substituted with sixteen
alkoxy groups (linear, branched and fluorinated alkoxy groups) were synthesized
from the corresponding tetraalkoxyphthalodinitriles. In the UV/Vis spectra of these
hexadecaalkoxy substituted phthalocyanines a bathochromic shift of the Q band up to 80 nm
relative to the unsubstituted phthalocyanines was observed. The hexadecaalkoxy
substituted phthalocyanines show excellent solubility in organic solvents. Systems
with long linear side chains exhibit unexpected low melting points
[7]
.
Electrochemistry plays an important role in our research group in the
characterization of the soluble macrocycles. Electronic properties of the
monomeric and polymeric complexes are characterized by cyclic voltammetry and
spectroelectrochemistry.
 Top of the Page
Top of the Page
 -electron heterocyclic aromatic
system derived from porphin. The more systematic name is [therefore]
tetraazatetrabenzoporphyrine. The annullation of further benzene units leads to
the naphthalocyanines 1,2-NcH
2
and 2,3-NcH
2
, respectively
(figure 1).
-electron heterocyclic aromatic
system derived from porphin. The more systematic name is [therefore]
tetraazatetrabenzoporphyrine. The annullation of further benzene units leads to
the naphthalocyanines 1,2-NcH
2
and 2,3-NcH
2
, respectively
(figure 1).
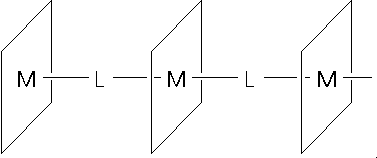
 = Pc, 1,2-Nc, 2,3-Nc, TBP, TPP
= Pc, 1,2-Nc, 2,3-Nc, TBP, TPP RT
= 2·10
-2
S/cm, without
external doping should be mentioned here
RT
= 2·10
-2
S/cm, without
external doping should be mentioned here
 Top of the Page
Top of the Page